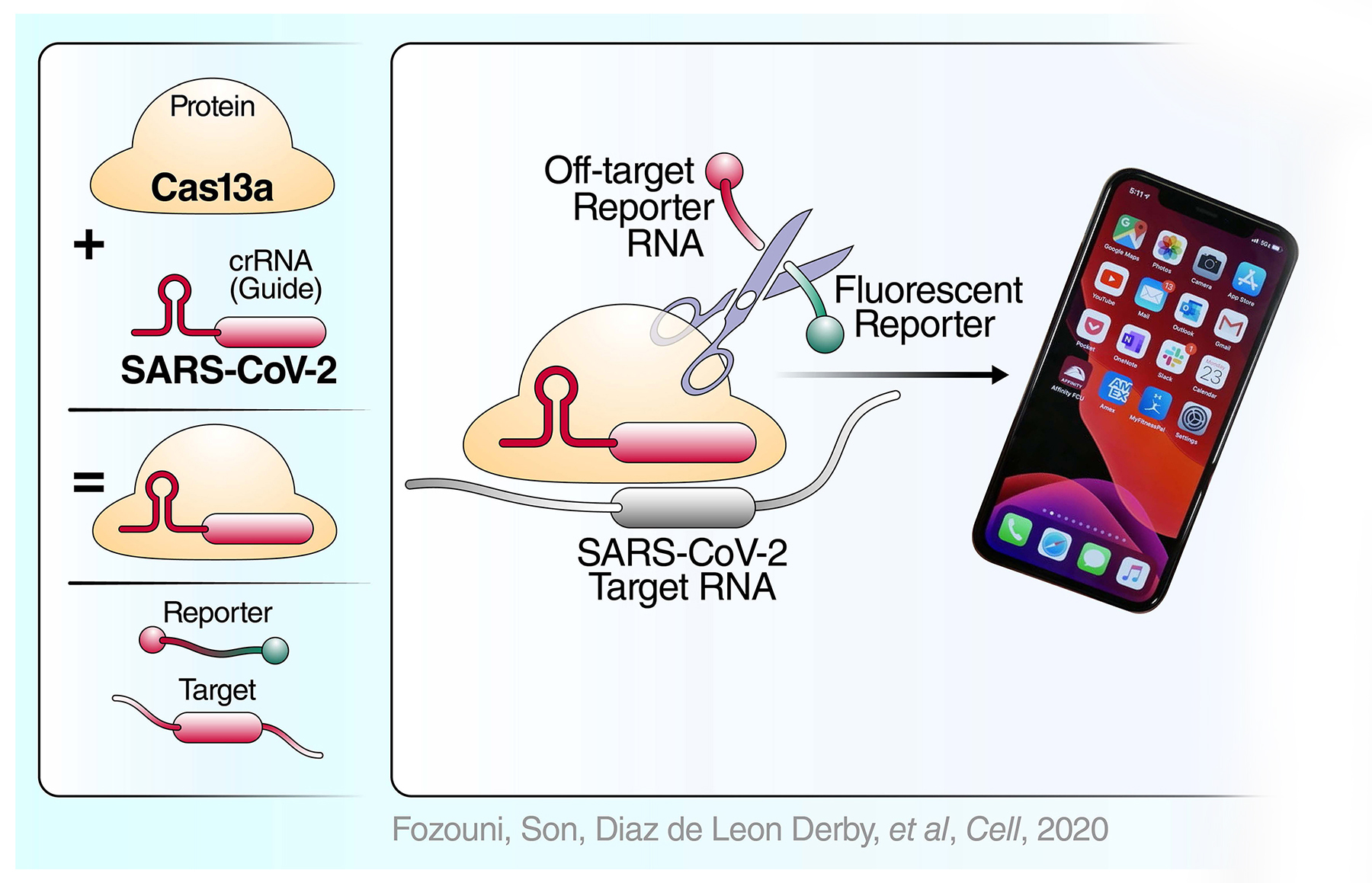
COVID-19 pandemic — applying our
best tools to a new virus
When news of a novel pathogenic coronavirus reached us, we knew that it was an “all hands on deck” situation. Two other coronaviruses, SARS and MERS, had already proven highly contagious and deadly.
We identified four areas where we could contribute: